
The peer-reviewed medical journal “Pharmacokinetics and Pharmacodynamics” has been published since 2004 and covers research on new and generic drugs. The journal publishes articles devoted to the study of experimental and clinical pharmacokinetics, preclinical pharmacodynamics, drug–drug interactions, pharmaceutical bioequivalence, therapeutic drug monitoring, and toxicity.
Journal publications will be useful to specialists studying the experimental pharmacokinetics of pharmacologically active compounds; clinical pharmacokinetics of new original drugs, including phase I clinical trials; biotransformation of various drugs; relationships between the pharmacokinetic and pharmacodynamic characteristics of drugs in experiments and clinics; biopharmaceutical aspects to create optimal dosage forms of drugs; bioavailability of generic drugs in experiments and bioequivalence in the clinic; and training of qualified specialists in the field of pharmacokinetics.
Since May 25, 2022, the journal "Pharmacokinetics and Pharmacodynamics" has been included in the list of Higher Attestation Commissions, peer-reviewed scientific publications in which the main scientific results of dissertations for the scientific degree of Candidate of Sciences and for the scientific degree of Doctor of Sciences are published (No. 2456 in the List). Scientific specialties and corresponding branches of science for which the publication is included in the List of Higher Attestation Commissions:
3.3.6. Pharmacology and clinical pharmacology
As of December 19, 2023, the HIGH CERTIFICATION COMMISSION under the Ministry of Science and Higher Education of the Russian Federation completed the categorization of scientific journals from the List of Higher Attestation Commissions into categories K1, K2, K3 in 2023. https://vak.minobrnauki.gov.ru/documents#tab=_tab:editions~
The journal "Pharmacokinetics and Pharmacodynamics" was included in the K1 category of the Higher Attestation Commission.
For reference:
To obtain admission to defend his/her candidacy and a doctoral dissertation, an applicant for an academic degree must publish a certain number of articles.
A graduate student in humanities is required to publish 3 articles, and applicants in other scientific fields are required to provide 2 published works.
Doctoral students must publish 15 and 10 scientific articles in the humanities and other sciences, respectively.
With the approval of the quartile system of journals of the Higher Attestation Commission, the following rules apply to Russian scientists:
- At least one article by a graduate student must be published in a journal related to K1 or K2;
- 5 articles of doctoral applicants must be published in publications from the first and second quartiles.
The audience of the journal: clinical pharmacologists, pharmacologists, clinicians, researchers, scientists, healthcare and drug supply organizers, and employees of Russian and foreign pharmaceutical companies.
The journal was registered with the Federal Service for Supervision of Communications, Information Technologies and Mass Communications (Roskomnadzor) on 02/04/2021, media registration certificate PI No. FS 77-80349.
Current issue
NEW DRUGS DISCOVERY
MECHANISM OF ACTION
PRECLINICAL PHARMACODYNAMICS STUDIES
METHODS OF PHARMACOKINETIC STUDIES
PHARMACOKINETIC RESEARCHES
SCREENING FOR NEW PHARMACOLOGICALLY ACTIVE COMPOUNDS
Announcements
2025-12-27
Новый этап фармакопейного контроля: Европейская фармакопея отказывается от теста на кроликах и утверждает безживотные методы
 | Страсбург, 18 декабря 2025 года — Европейская дирекция по качеству лекарственных средств и здравоохранения (EDQM) объявила о историческом изменении, которое знаменует новую эру инноваций и устойчивого развития в фармацевтическом контроле. С 1 января 2026 года классический тест на пирогены на кроликах будет полностью исключен из текстов Европейской фармакопеи (Ph. Eur.). Его место займут современные, научно обоснованные и не требующие использования животных методы. |
2025-12-12
Рецензия на книгу: «Руководство по этике научных исследований» / под общей ред. А.Л. Хохлова. — М.: Изд-во ОКИ, 2026. — 764 с.
 | Перед научным сообществом стоит непростая задача: не отставая от стремительного развития наукоёмких технологий — от искусственного интеллекта и генной инженерии до синтетической биологии, — одновременно отвечать на порождаемые ими принципиально новые этические вызовы. |
2025-12-07
Установление и внедрение стандартов для препаратов с узким терапевтическим индексом
 | В клинической фармакологии используется термин "лекарственный препарат с узким терапевтическим диапазоном". В данном ключе интересен опыт FDA и центра оценки и исследований лекарственных средств (CDER) в отношение данной группы лекарств. |
2025-11-26
Руководство по замене или отмене испытаний на животных при контроле качества биологических лекарственных препаратов
 | Экспертный комитет Всемирной организации здравоохранения (ВОЗ) по биологической стандартизации (ECBS) утвердил «Руководство по замене или отмене испытаний на животных при контроле качества биологических лекарственных препаратов» на заседании, проходившем 13–16 октября 2025 года. Окончательная версия руководства размещена на сайте ВОЗ для предварительного ознакомления, а официальная публикация будет включена в Серию технических отчётов ВОЗ в 2026 году. |
2025-10-30
FDA предложило отказаться от большинства сравнительных клинических исследований эффективности при регистрации биоподобных лекарственных препаратов
 | Американское Управление по санитарному надзору за качеством пищевых продуктов и медикаментов (FDA) опубликовало проект руководства «Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Updated Recommendations for Assessing the Need for Comparative Efficacy Studies» (октябрь 2025). |
2025-10-15
FDA официально внедрила новую версию международных стандартов Good Clinical Practice — ICH E6(R3)
 | В сентябре 2025 года Управление по контролю за продуктами и лекарствами США (FDA) опубликовало финальное руководство E6(R3) Good Clinical Practice (GCP), тем самым утвердив его как национальный норматив для всех клинических исследований, проводимых под юрисдикцией США. Документ завершает переход от международного уровня к национальному: теперь все исследования, подаваемые в FDA, должны соответствовать именно версии R3. |
2025-10-12
Научный центр экспертизы Минздрава выпустил рекомендации по доклиническим исследованиям оригинальных ЛП
 | Научный центр экспертизы средств медицинского применения Минздрава выпустил практические рекомендации по доклиническим исследованиям (ДКИ) оригинальных лекарственных препаратов (ЛП). |
2025-10-06
FDA обновил руководства по разработке дженериков: ключевые изменения и возможности для фармпроизводителей
 | FDA опубликовал актуализированные Product-Specific Guidances (PSG) — документы, определяющие требования к разработке и регистрации дженериков. Эти руководства помогают производителям разрабатывать препараты, эквивалентные оригинальным лекарственным средствам (RLD), и обеспечивать их биоэквивалентность, безопасность и эффективность. |
2025-09-27
Кабмин поручил разработать систему скрининга лекарств с использованием модели «человек на чипе»
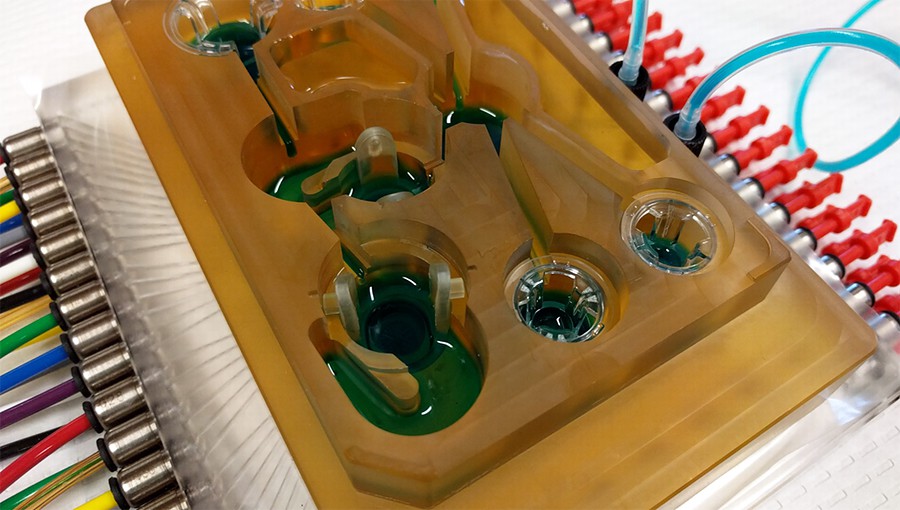 | Это следует из постановления правительства, которое вносит изменения в Федеральную научно-техническую программу развития генетических технологий до 2030 года. «Создание системы высокопроизводительного скрининга лекарственных препаратов с использованием модели "человек на чипе"» является теперь одной из задач программы. |
2025-09-27
Демис Хассабис: ИИ способен сократить сроки открытия лекарств до нескольких месяцев
 | Глава DeepMind рассказал в интервью Bloomberg, как ИИ меняет фармацевтическую науку, минимизируя неудачи, снижая стоимость исследований и открывая путь к персонализированной медицине. |
| More Announcements... |













































